Featured Post
Who Can Own a Medical Spa?

Show your committment to patient safety, legal compliance and community over competition.
AmSpa members receive preferred pricing on all AmSpa live and virtual trainings.
Get the latest news and information about safe, legal practice in medical aesthetics directly in your inbox.
Get access to med spa laws, in-person and online training and more!

Legal
By Adam Reinebach, Chief Executive Officer, American Med Spa Association (AmSpa)New Jersey is at a crossroads.With the recent expiration of ...

Legal
By Madilyn Moeller, Marketing Content Coordinator, American Med Spa Association (AmSpa)A few lines in a state bill can fundamentally change ...

Business
By Adam Reinebach, Chief Executive Officer, American Med Spa Association (AmSpa)Since stepping into the CEO role at AmSpa, I’ve spent ...

AmSpa Events
We’re thrilled to share that Thursday pre-show deep-dive sessions, Wake Up and Learns and Lunch and Learns and the ever-popular ...

Marketing
By AestheticsPro For medical spa owners looking to reach their clients in the most...
Top Tags
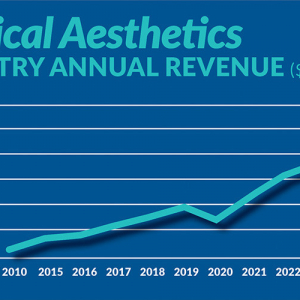
January 24, 2023
Figure displays medical aesthetics industry annual revenue ($ in billions). By Madilyn Moeller Day-to-day life...

February 24, 2021
By Bradford E. Adatto, Partner, ByrdAdattoIntravenous (IV) therapy has been used to provide nutrition and...

September 21, 2023
By Patrick O'Brien, JD, General Counsel, American Med Spa Association [AmSpa first published a...
July 19, 2023
By Patrick O'Brien, General Counsel, American Med Spa Association (AmSpa) UPDATE #2 (2/08/2024): Since...