Featured Post
Who Can Own a Medical Spa?

AmSpa Events
AmSpa’s Women in Aesthetics Leadership Conference (WALC) is a transformational experience designed exclusively for accomplished women in medical aesthetics, offering ...

Consumers
Walk into any modern med spa, and you're likely to find PAs taking a prominent role in patient care—conducting consultations ...

Business
By OptimantraRunning a med spa is an exciting business, full of opportunities for growth and innovation. However, there’s a hidden ...

AmSpa Events
The first phase of growth is make or break for new businesses. Half (49.4%) of small businesses fail within the ...

Marketing
By AestheticsPro For medical spa owners looking to reach their clients in the most...
Top Tags
January 24, 2023
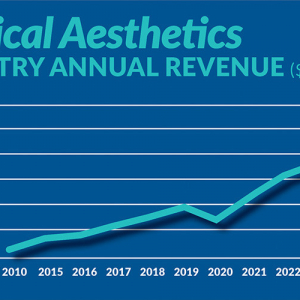
Figure displays medical aesthetics industry annual revenue ($ in billions). By Madilyn Moeller Day-to-day life...

February 24, 2021
By Bradford E. Adatto, Partner, ByrdAdattoIntravenous (IV) therapy has been used to provide nutrition and...

September 21, 2023
By Patrick O'Brien, JD, General Counsel, American Med Spa Association [AmSpa first published a...
July 19, 2023
By Patrick O'Brien, General Counsel, American Med Spa Association (AmSpa) UPDATE #2 (2/08/2024): Since...