Featured Post
Who Can Own a Medical Spa?

Show your committment to patient safety, legal compliance and community over competition.
AmSpa members receive preferred pricing on all AmSpa live and virtual trainings.
Get the latest news and information about safe, legal practice in medical aesthetics directly in your inbox.
Get access to med spa laws, in-person and online training and more!

Marketing
Local SEO for Med Spas: How to Get Found (and Booked) by Clients in Your AreaBy Jennifer Orechwa, Salt MarketingEvery ...

Equipment
By EarthliteFor years, many med spas viewed treatment tables as simple pieces of furniture—necessary, yes, but hardly strategic. Today, that ...

Marketing
By DarwillMany med spas put a lot of effort into attracting new clients, but often lose them after just one ...

Clinical
With Molly Muecke, RN, MSN, NP-CMicroneedling is a popular aesthetic treatment offered in 79% of med spas (2024 Medical Spa ...


Marketing
By AestheticsPro For medical spa owners looking to reach their clients in the most...
Top Tags
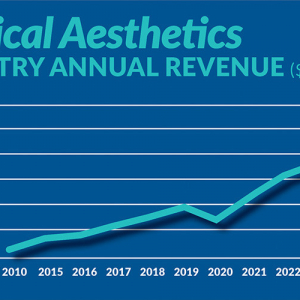
January 24, 2023
Figure displays medical aesthetics industry annual revenue ($ in billions). By Madilyn Moeller Day-to-day life...

February 24, 2021
By Bradford E. Adatto, Partner, ByrdAdattoIntravenous (IV) therapy has been used to provide nutrition and...

September 21, 2023
By Patrick O'Brien, JD, General Counsel, American Med Spa Association [AmSpa first published a...
July 19, 2023
By Patrick O'Brien, General Counsel, American Med Spa Association (AmSpa) UPDATE #2 (2/08/2024): Since...