
April 24, 2024
FTC Approves Rule Against Non-competes
By Patrick O’Brien, JD, General Counsel, American Med Spa Association (AmSpa) This week, the U.S. Federal Trade Commission (FTC) ...
Legal, business and clinical training resources to build legally compliant, profitable and safe medical aesthetic practices.
Become A MemberRegistration Now Open for Medical Spa Show 2024
State-by-State Legal Summaries
AmSpa's legal summaries allow you to quickly reference the laws governing medical spas in your state. They are a list of commonly asked questions researched and prepared for easy use by a team of skilled lawyers at ByrdAdatto Law Firm.
Treatment Delegation Tables
AmSpa Plus members can access this easy-to-use resource outlining Who Can Do What in their state, covering 21 medical aesthetic treatments across 6 professional titles.
Legal Updates
AmSpa Plus members also get alerts on potential bills affecting med spa laws in their state.

State-by-State Legal Summaries
AmSpa's legal summaries allow you to quickly reference the laws governing medical spas in your state. They are a list of commonly asked questions researched and prepared for easy use by a team of skilled lawyers at ByrdAdatto Law Firm.
Treatment Delegation Tables
AmSpa Plus members can access this easy-to-use resource outlining Who Can Do What in their state, covering 21 medical aesthetic treatments across 6 professional titles.
Legal Updates
AmSpa Plus members also get alerts on potential bills affecting med spa laws in their state.

Online and On-demand Medical Aesthetic Education
Grow your practice with AmSpa's Learning Center to improve your legal compliance, business processes and clinical skill. Register for upcoming live webinars or watch more than 180 recorded webinars dealing with medical aesthetic law, business processes, marketing, and treatments.
Learn new techniques with our online AmSpa Masters courses, build up your anatomy knowledge with the AIA Virtual Anatomy Lecture Series or find out how to run a legal and profitable practice with the Virtual Boot Camp.
All the knowledge of industry-leading trainers and all the comforts of home.

Online and On-demand Medical Aesthetic Education
Grow your practice with AmSpa's Learning Center to improve your legal compliance, business processes and clinical skill. Register for upcoming live webinars or watch more than 180 recorded webinars dealing with medical aesthetic law, business processes, marketing, and treatments.
Learn new techniques with our online AmSpa Masters courses, build up your anatomy knowledge with the AIA Virtual Anatomy Lecture Series or find out how to run a legal and profitable practice with the Virtual Boot Camp.
All the knowledge of industry-leading trainers and all the comforts of home.

Members get preferred pricing on all of AmSpa's industry-leading training events.
Medical Spa & Aesthetic Boot Camps give you the tools to build and run a legally compliant and profitable medical spa practice. Click here to grow your business.
The Academy for Injection Anatomy has a clinical training for every experience level of injector so you can provide the safest treatments and best results for your patients. Click here to increase your skill level.
The Medical Spa Show brings the biggest and best of medical aesthetics together into one community. Build your practice, level-up your skillset, meet with the top brands in the industry and make connections that will last a lifetime. Click here to find your community.

Members get preferred pricing on all of AmSpa's industry-leading training events.
Medical Spa & Aesthetic Boot Camps give you the tools to build and run a legally compliant and profitable medical spa practice. Click here to grow your business.
The Academy for Injection Anatomy has a clinical training for every experience level of injector so you can provide the safest treatments and best results for your patients. Click here to increase your skill level.
The Medical Spa Show brings the biggest and best of medical aesthetics together into one community. Build your practice, level-up your skillset, meet with the top brands in the industry and make connections that will last a lifetime. Click here to find your community.

Get Guidance from Top Legal Minds
AmSpa members receive a complimentary 15-20 minute consult call with ByrdAdatto. The goal for the consult is for ByrdAdatto to learn more about your legal needs and help diagnose whether there are any current business or health care compliance issues that need legal attention.

Get Guidance from Top Legal Minds
AmSpa members receive a complimentary 15-20 minute consult call with ByrdAdatto. The goal for the consult is for ByrdAdatto to learn more about your legal needs and help diagnose whether there are any current business or health care compliance issues that need legal attention.

AmSpa Connect
AmSpa members can network, share stories and gain advice from med spa professionals just like them with our members-only online community, AmSpa Connect. Find like-minded people based on state or job title, or just chat with the full member community.
AmSpa Member Lounge on Facebook
Members can also network with each other conveniently in our members-only Facebook group.

AmSpa Connect
AmSpa members can network, share stories and gain advice from med spa professionals just like them with our members-only online community, AmSpa Connect. Find like-minded people based on state or job title, or just chat with the full member community.
AmSpa Member Lounge on Facebook
Members can also network with each other conveniently in our members-only Facebook group.


April 24, 2024
By Patrick O’Brien, JD, General Counsel, American Med Spa Association (AmSpa) This week, the U.S. Federal Trade Commission (FTC) ...

April 17, 2024
By Patrick O’Brien, JD, General Counsel, American Med Spa Association (AmSpa) There appears to be a national wave of ...

April 1, 2024
By Michael Meyer, Content Manager, American Med Spa Association (AmSpa) The American Med Spa Association (AmSpa) has announced a ...
Get preferred pricing for online and in-person training events, only with an AmSpa Membership.
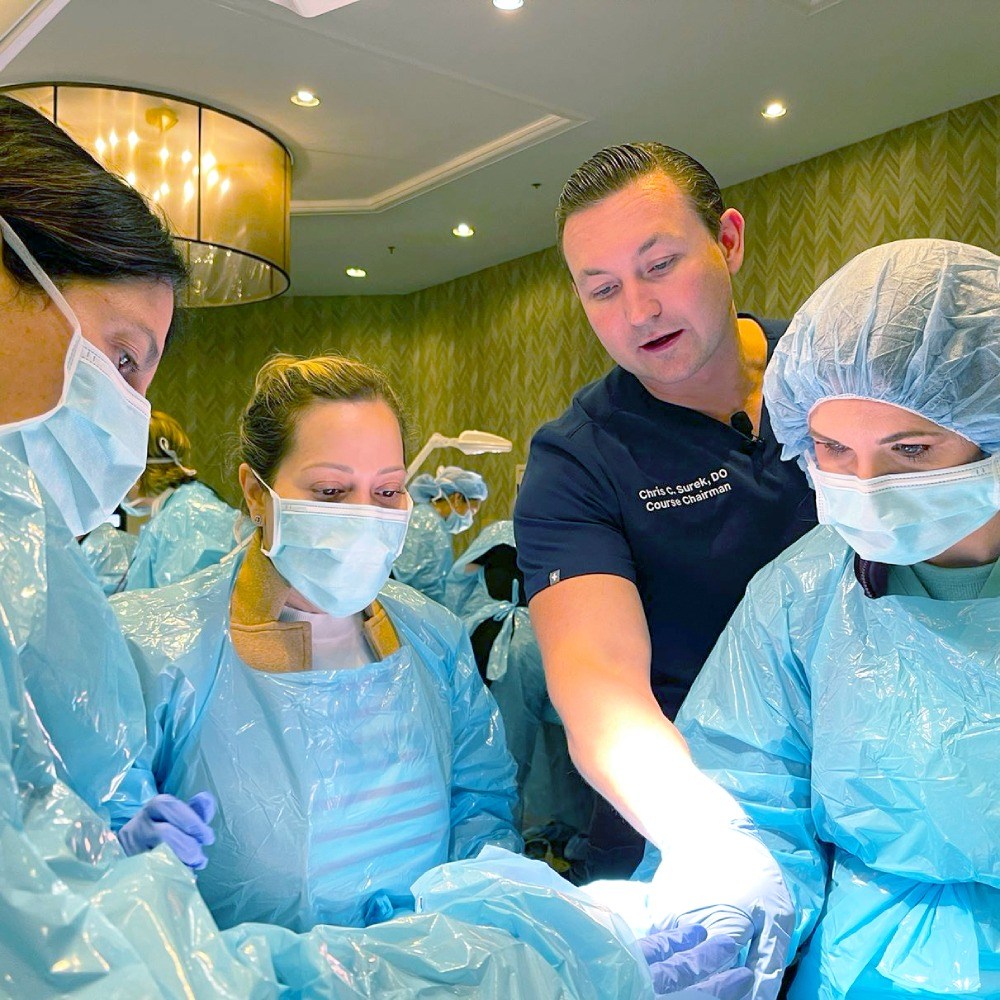
Advanced anatomy and technique training for neuromodulator and filler injections.

Learn medical spa marketing tactics, business strategies and get a breakdown of med spa law.

Inject more confidently by seeing the anatomy below the skin. Ultrasound helps you visualize the anatomy of your patient, guiding you to safer injections and better results.
Read about recent events, essential information and the latest industry news.
The Federal Trade Commission narrowly voted Tuesday to ban nearly all noncompetes, employment agreements that typically prevent workers from joining competing businesses or launching ones ...
Ginille Brown’s plan was to work in family medicine or an emergency room. But when someone suggested looking into a position at a med spa ...
Doctors for Providers has renewed its collaboration with the American Med Spa Association (AmSpa) as a Silver Vendor Affiliate. Doctors for Providers is a leading ...
AmSpa Members save 10% on job postings! Check your welcome email or contact us for your exclusive promo code.
Visit your state's chat community to connect with med spa professionals in your state, and to find easy links to your state medical aesthetic legal summary.
Visit your professional chat community to connect with med spa professionals across the country who share your job title and have stood in your shoes.